During the 24th IUCr 2017 Congress, a semi-formal program will run parallel to the scientific sessions. This is the first time such an activity is being conducted in an IUCr Congress
It will include:
- discussions and roundtables on education, outreach and capacity building;
- seminars on general interest topics;
- contests for research grants for youngsters;
- real and operational single-crystal XRD lab to collect your own data;
- exhibitions;
- Dragons’ Den contest (registered paid-up students only) – win INR 1,50,000 to support your dream research project. Submissions now closed.
- and much more …
For further information on the parallel program please contact M Zema on zema@iucr2017.org
Timetable
| Date | Location | Parallel Program |
| Full duration of the Congress | HICC main entrance | Mega Structure Build. Inauguration at 10:15 on Tuesday, 22 August |
| Full duration of the Congress | Ground floor Lobby | MicROCKScopica photo exhibition |
| 21 to 26 August, 2017 | Organisers Office 2 | Rigaku XtaLab |
| Tue, 22 August, 10:30-13:00 | MR G.02-G.03 | Salt of the Earth |
| Tue, 22 August, 15:00-17:30 | MR G.02-G.03 | Academia/Industry/Government Collaborations in Drug Design and Drug Development |
| Wed, 23 August, 10:30-13:00 | MR G.02-G.03 | IUPAP-IUCr LAAMP kick-off meeting |
| Wed, 23 August, 10:30-13:05 | MR G.02-G.03 | LAAMP kick-off meeting |
| Wed, 23 August, 15:00-15:30 | MR G.02-G.03 | Towards a global community: the IUCr Associates Programme |
| Wed, 23 August, 15:30-17:30 | MR G.02-G.03 | Symmetry in Indian Art |
| Thu, 24 August, 10:30-13:00 | MR G.02-G.03 | Crystallography in emerging nations: projects for a sustainable development of education and research infrastructure in Africa |
| Thu, 24 August, 15:00-15:30 | MR G.02-G.03 | Towards a global community: the IUCr Associates Programme |
| Thu, 24 August, 15:00-17:30 | MR G.01 | Dragons’ Den |
| Fri, 25 August, 10:30-13:00 | MR G.01 | Commemorative symposium in honor of Howard Flack |
| Fri, 25 August, 15:00-15:30 | MR G.02-G.03 | Towards a global community: the IUCr Associates Programme |
| Fri, 25 August, 15:30-17:30 | MR G.02-G.03 | Crystallography in emerging nations: LACA |
| Sat, 26 August, 10:30-13:00 | MR G.01 | Dragons’ Den |
| Sat, 26 August, 15:00-17:30 | MR G.02-G.03 | Crystallography in emerging nations: the role for development in the BRICS countries |
| Sat, 26 August, 15:00-17:30 | MR G.01 | Symposium in honor of William L Duax |
Mega structure build
Supported by:
- Where: HICC main entrance
- When: entire duration of the Congress
- Sponsors: Department of Science and Technology, Govt. of India; Austrian Cultural Forum
The world’s largest crystal structure model, which has been entered in the Guinness Book of Records, is being brought to Hyderabad from Vienna. Its creator, Robert Krickl, will be with us between 21 and 24 August and will help personally explain the model.

MicROCKScopica art exhibition
- Where: Ground floor lobby
- When: entire duration of the Congress
- Organizer: IUCr Commission on Art and Cultural Heritage

Award-winning photomicrographs of rocks and crystals by Bernardo Cesare (petrologist at U. Padua, Italy) will be displayed. Cesare’s photos have already been shown in the IYCr2014 calendar and have been exhibited in Italy, France, Germany, Hungary, Poland, Spain, Switzerland, UK and USA. Some examples are available at http://microckscopica.altervista.org/en/
Rigaku XtaLab
- Where: Organizer Suite 2
- When: 21 to 26 August, 2017
- Tutors and operators: Rigaku technical staff
A real X-ray diffraction lab will be in operation for participants to collect their own data. The Rigaku XtalLab will be equipped with benchtop single-crystal (XtaLAB mini II) and powder (MiniFlex) X-ray diffractometers. Participants will then have the opportunity – with IUCr Journal editors on hand – to publish their results in an IUCr publication during the Congress. Why not bring your own crystal and take away a published article?!
Salt of the Earth
- Where: MR G.02-G.03
- When: Tuesday, 22 August, 10:30 – 13:00
- Chair: Gautam R. Desiraju (IISc, Bangalore)
- Speakers: R. Krickl, D. Joshi, A.M. Glazer, A. R. Oganov, A. Das
150 minute session on various aspects of this unique compound, sodium chloride, NaCl, common salt, that is so important in crystallography and also elsewhere. The motivation for this special session is the giant NaCl crystal structure model, the largest such model for any crystal structure, in IUCr2017. This model has made it to the Guinness Book of Records.
The model will be inaugurated at 1015 hours
The session will begin at 1030 hours
Chair: G.R. Desiraju (IISc, Bangalore).
Speakers:
-
Robert Krickl (Vienna) has designed and constructed the model and he will be talking to us about the model itself and his recollections on the subject, and the issues involved in making and exhibiting it, and the special arrangements that were made in transporting the model to India. His talk title “Communicating Crystallography – the world record crystal structure model”
-
Dinkar Joshi(Mumbai) will be speaking about the Salt March of 1930, or what Indians refer to as the Dandi March, in which Mahatma Gandhi broke the law and collected salt from the sea in Gujarat on our western shoreline. Why did Gandhi select salt for his ‘satyagraha’from the 11 issues he placed before Lord Irwin, the Viceroy of India? Why was the salt tax important to the British? His talk title “Salt is Supreme – Gandhi’s Dandi March (1930)”.
-
Mike Glazer (Oxford) will talk about the Braggs, father and son and how they determined the crystal structure of NaClin 1912, the first ever crystal structure to be determined from an analysis of its X-ray diffraction pattern. Why did Lawrence Bragg select NaCl and how is his method of structure solution different from what crystallographers use today? What was the contribution of William Bragg? His talk title “The two Braggs and common salt”.
-
Artem Oganov (Skoltech, Russia and SUNY, USA) will speak about the great importance of the NaCl structure in the context of solid state chemistry. Why is this structure so common for an MX compound? What makes it so versatile? Why is still a topic of research interest, more than a century after it was determined? His talk title “The NaCl structure type in solid state chemistry and crystallography”.
-
Amitava Das (CSMCRI, Bhavnagar) will tell us about the importance of NaCl in the commercial and industrial perspective in India with respect to quality and yield. He will also provide a glimpse about the importance of extracting potash fertiliser from sea water. The relevance of potash extraction technologies for alcohol distilleries in India will be highlighted. His talk title “Strategic importance of sodium chloride and potassic chemicals for India”.
Academia/Industry/Government Collaborations in Drug Design and Drug Development
- Where: MR G.02-G.03
- When: Tuesday, 22 August, 15:00-17:30
- Chair: Girish Sahni, Director-General, CSIR India
- Speakers: Gautam R. Desiraju (IISc), Stephen R. Byrn (Purdue), Girish Sahni (CSIR), Rakesh K. Khandal (India Glycols), A. Venkateswarlu (DRILS)
This 150-minute session will highlight academia/industry/government interactions in the area of solid state pharma and novel crystal forms for innovator and generic companies.
Speakers:
-
G. R. Desiraju (IISc, Bangalore) will present a survey of solid forms that are of significance in the development of new drugs and why they are expected to show promise in the near future. Salts, cocrystals, solvates and amorphous forms are different solid state variations of a drug. Any of these forms is capable of existing in polymorphic modifications. All this greatly increases the structural landscape which the drug inhabits: this is turn is expected to be the key to property modulation. Notable among the properties that can be modified are solubility and permeability, thereby affecting bioavailability. The core inputs for this and related activity arises from the field of crystal engineering and the possibilities for logic driven solid state synthesis through supramolecular heterosynthons. His talk title, “API solid forms and the crystal structure landscape”.
-
S. R. Byrn (Purdue University, USA), will summarize the regulatory science of solid-state chemistry. The FDA defines regulatory science as the science of developing new tools, standards, and approaches to address safety, efficacy, and quality. The role of solid-state chemistry will be outlined. The Q1/Q2/Q3 approach to determine equivalence will be used: Q1 – same ingredients, Q2 – same amounts of ingredients, and Q3 has a broader meaning related to same structure, microstructure, and physical/chemical properties. From the perspective of solid-state chemistry, Q3 would mean same solid-state structure. The determination of Q3 involves a range of crystallographic studies including powder diffraction, single crystal studies, and pair distribution function analysis. The title of his talk is: Regulatory Science and the Solid State Chemistry of Drugs.
-
G. Sahni (CSIR Headquarters, New Delhi) will describe his experiences with drug design based on macromolecular crystallography. Critical and intricate structural details associated with streptokinase, a bacterial thrombolytic protein, were gleaned from X-ray and solution studies to design newer drug candidates with smarter and clinically useful functions. Crystallography is important in drug design and redesign, as well as in gaining deep mechanistic insights but when the resolution is limited, the available structures do not represent the full complement of polypeptide partners relevant to the biology of the multi-component system. Streptokinase represents one such example. His talk title: Quasi-structural studies in the redesign of a clot-buster protein drug.
-
R. K. Khandal (India Glycols) Drug discovery involves a large number of steps before a desired drug molecule is introduced in the market. Continuous research and development efforts must come up with novel and innovative molecules for the purpose. One of the key aspects of drug discovery pertains to the development and validation of chemical processes to manufacture the desired molecule. Often, industry units keep a watch on the molecules that are going off-patent. By the time they are allowed to manufacture products that are already off-patent, they must be ready before-hand with a cost effective process. India is already in a leadership position as far as generics are concerned. In the present era of competition, Indian industry would need technical support from organisations like CSIR. His talk title, “Success factors for drug processing and manufacture.”
-
A. Venkateswarlu (DRILS, Hyderabad), will present a high level SWOT analysis of pharmaceutical cocrystals and their potential role in design and development of therapeutics. The emerging impact of cocrystals as alternate solid forms with pharmaceutical applications, both in the drug substance and the drug product, presents itself as a powerful opportunity to address unmet medical needs. Typically, the landscape of new chemical entities (NCEs) and active pharmaceutical ingredients (APIs) has been dominated by solid forms, involving either the native chemical forms or salts, solvates and corresponding polymorphs thereof. Cocrystal forms of such NCEs or APIs as novel compositions of matter offer not only IP potential but also non-obvious therapeutic profile coupled with means to favorably impact the life cycle management. His talk title, “Pharmaceutical Cocrystals: a SWOT Perspective”.
Towards a global community: the IUCr Associates Programme
- Where: MR G.02-03
- When: Wednesday, 23 August, 15:00-15:30; Thursday, 24 August, 15:00-15:30; Friday, 25 August, 15:00-15:30
- Chair: Jonathan Agbenyega (IUCr)
The IUCr Associates Programme will launch officially during the 2017 IUCr Congress and General Assembly. By joining the Associates Programme you can be a part of the IUCr. The IUCr is involved in many charitable activities including supporting students to attend meetings internationally, a Visiting Professor scheme and building crystallography capacity around the world. To learn more about the Associates Programme and our other activities please join this session.
Kick-off meeting of LAAMP, an IUPAP-IUCr Project within the ICSU Grants Programme 2016-2019
- Where: MR G.02-G.03
- When: Wednesday, 23 August, 10:30-13:00
- Chairs: Michele Zema (IUCr) and Sandro Scandolo (IUPAP)
- Speakers: Sandro Scandolo (IUPAP; UNESCO-ICTP; LAAMP Executive Committee) and Michele Zema ((IUCr; U. Pavia; LAAMP Executive Committee)); Andrea Lausi (ELETTRA; http://www.lightsources.org/; LAAMP Brochure Committee); Jean-Paul Ngome Abiaga (UNESCO-IBSP); Gihan Kamel (SESAME); others to be confirmed.
The project “Utilisation of Light Source and Crystallographic Sciences to Facilitate the Enhancement of Knowledge and Improve the Economic and Social Conditions in Targeted Regions of the World” (abbreviated into Lightsources for Africa, the Americas and the Middle East Project, LAAMP), will enhance advanced light source and crystallographic sciences in three global regions by developing regional plans to grow and enhance understanding of light source techniques and crystallography in these regions.
LAAMP kick-off meeting
- Chairs: Michele Zema (IUCr), Sandro Scandolo (IUPAP)
Wednesday, 23 August, 10:30-13:05 hrs - Room: MR G.02-G.03
- Speakers: Sandro Scandolo (IUPAP; UNESCO-ICTP; LAAMP Executive Committee) and Michele Zema (IUCr; U. Pavia; LAAMP Executive Committee); Andrea Lausi (ELETTRA; http://www.lightsources.org/; LAAMP Brochure Committee); Jean-Paul Ngome Abiaga (UNESCO-IBSP); Gihan Kamel (SESAME)
The project “Utilisation of Light Source and Crystallographic Sciences to Facilitate the Enhancement of Knowledge and Improve the Economic and Social Conditions in Targeted Regions of the World” (abbreviated into Lightsources for Africa, the Americas and the Middle East Project, LAAMP), will enhance advanced light source and crystallographic sciences in three global regions by developing regional plans to grow and enhance understanding of light source techniques and crystallography in these regions.
- 10:30-10:35, M.L. Hackert (IUCr President), Welcome and Opening remarks
- 10:35-10:50, D. Nyanganyura (ICSU-ROA Director),The 2016-2019 ICSU Grants Programme
- 10:50-11:05, M. Zema and S. Scandolo (LAAMP EC), Introduction to LAAMP
- 11:05-11:20, J.P. Ngome Abiaga (UNESCO IBSP Deputy Director), Light sources and the sustainable development goals
- 11:20-11:30, S. Scandolo (IUPAP, UNESCO ICTP), LAAMP in the context of the IUPAP outreach initiatives
- 11:30-11:40, M. Zema (IUCr), LAAMP in the context of the IUCr outreach initiatives
- 11:40-11:55, A. Lausi (ELETTRA), The role of partnering AdLSs and the LAAMP brochure
- 11:55-12:10, A. Craievich (U. São Paulo), Brazilian synchrotron light sources: Development and contribution to scientific integration in Latin America
- 12:10-12:25, R. Garratt (São Carlos Institute of Physics), A brief history of protein crystallography in Brazil – the central role of a synchrotron facility
- 12:25-12:40, G. Kamel (SESAME), The impact of SESAME to scientific development in the Middle-Eastand the neighbouring regions
- 12:40-12:50, A. Roodt (U. Bloemfontein), The African Light Source: dreaming of a brighter future
- 12:50-13:05, M. Zema and S. Scandolo (LAAMP EC), Roundtable and Concluding remarks
Symmetry in Indian Art
- Where: MR G.02-G.03
- When: Wednesday, 23 August, 15:30-17:30
- Chair: Jean Marc Castera (tbc)
- Speaker: Poosapati Parameshwar Raju
Renowned Indian artist Poosapati Parameshwar Raju will present a seminar on “The Mechanism of Splendour”, demonstrating the area of dynamic approach to the grid and its manifestation in visualizing a narrative, with examples in design and temple architecture.
Parameshwar’s creativity as an artist, calligrapher and his expertise in communication, documentation and publication design, is celebrated across the globe. In his work, we witness the phenomenon of a new genre that could be termed as iconic calligraphy. The artist engages with a distinguished form of expression that traverses both traditional and contemporary terrains. His images are offspring of an epistemological discernment and the minimalist’s aesthetic, ensuing from his personalized research of iconography, signs, and symbols inherent in the Indian ceremonial culture. He has been practicing for the last 30 years. Parameshwar is one of the trustees of Jagdish and Kamla Mittal Museum of Indian Art, Hyderabad. His book on designs and documentation work for the museums have set new standards for museum publications and is being followed by leading museums all over the world. He is in the process of setting up a foundation for the arts for generating work for artisans with his design forms and traditional art and craft, for the self-reliance of artisans, specific to Indian regions.
“The Mechanism of Splendour” – Introduction

I am more comfortable with the traditional design and its base, which I stumbled upon once when my grandfather Sri Poosapati Raghav Raju corrected me during our conversation regarding Indian art anddesign, inside the Ellora caves. He was a traditional artist who had the knowledge of the traditional yantra which was used knowingly or unknowingly by our traditional artists and temple builders. I would like to share this knowledge to help create an understanding for some very coherent points of discussion on design by using the principles of image formation, symmetry and balance, for which the grid in Indian design is such a versatile device. The circle when divided evokes creation composition of the universe. It is divided into two by a vertical line and then into four by a horizontal line, the two lines symbolizes respectively the elements of fire (masculine) and water (feminine) while the resulting four areas are likened to the four corners of the universe and the four directions – NE, SE, SW and NW.

The line is the support of the composition on which one builds the image corresponding to the elements and to the gods. Straight lines are like the rays of light as the creator divided the world. From these intersecting lines a square or rhombus is formed and the diagonals representing the winds. The points are added. A square is formed – This square is Ganesha. The lines referred as lines of light conjures an image of brilliance something like a crystal and not a blank space. The space is seen as full of energy & light.
In truth space is not empty, but alive with lines of light crisscrossing the space. Silently there happens a transformation of shapes to forms and forms to image. At the intersection of these lines the points are not isolated and meaningless but are a field of consciousness linked to it and each reflects or attracts the others. One can see a lot of bright spots which look like gems and it was called ‘Indra’s net of jewels’. These jewels are points of energy which identify places which are visible to the eye in a given space.
When we place our presence in these points we see ourselves as part of a whole, interdependent to lighten the presence giving it a character. This is dignity & divinity, which in turn takes us to a thought of a divine enlightened being, whose behavior defies common-sense and the limits of time & space.
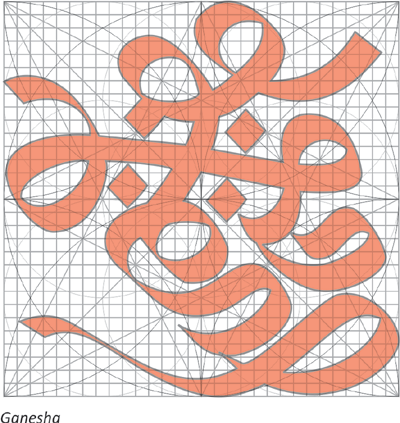
The ‘khila–pinjara’ must be correct for it binds & energizes the space and links to its surrounding spaces. The next step in the creation of image is to draw a grid which complements the khila-pinjara. This grid is a matrix of five lines by five- making 16 identical squares or rectangles intersected by two diagonals. This recalls the original creation of the universe when purusha the cosmic form sacrificed himself into sixteen parts. Each line symbolizes an element-vertical lines symbolizes fire, horizontal lines symbolizes water and diagonal lines symbolize wind.
The bottom row of four squares, from the foot to the knee, represents earth. The next, from the knee to navel, represents the reproductive energy. The third from navel to the neck is the area of the life–breath, and the top from neck to crown is the area of divine light. This grid is then divided into five fields. Each field corresponding to one of the five hierarchical realms of the universe, which in descending order are the realms of Gods & the secondary divinities; the living being.
We start with bindu; the basic element. As this bindu expands in linear space, it forms the circle and when it expands in space we call it a sphere. These two are basic forms. This bindu is also an intersection point of the rays of light. When three lines cross at a point and move away from each other we get triangular spaces and these triangular spaces are basic. When it expands in space it forms a prism.
In the traditional context Shiva and Shakti are formed and when these overlap a shatkona ( hexagon ) is formed, also called the Akarshani Yantra. With these triangles, a square is formed which represents Ganesha. The circle represent Surya and the disc represents Vishnu. Coming back to the bindu, we divide the space into multiples of 5 or 9.
The square space has four points and the center has a concentrated source of energy which attracts these points. So we have two diagonal rays of light formed. The horizontal and the vertical –the water and fire lines are added to these. Then we join the remaining points and where ever these rays intersect we call them hot points or points of light, the jewels of Indra. These are points which attract the eye. This is the basic grid on which design is done, so that we work on the form which makes one see in totality.
Crystallography in emerging nations: projects for a sustainable development of education and research infrastructure in Africa
- Where: MR G.02-G.03
- When: Thursday, 24 August, 10:30-13:00
- Chair: Michele Zema (IUCr), Claude Lecomte (U. Lorraine, Nancy)
- Speakers and Panelists: Claude Lecomte (Chair of the IUCr Crystallography in Africa initiative), Jean-Paul Ngome Abiaga (UNESCO-IBSP), Sandro Scandolo (UNESCO-ICTP), Daniel Nyanganyura (ICSU-ROA), Andreas Roodt (Chair of the Steering Committee for the African Crystallographic Association).
This session will present projects and activities for a sustainable development of education and research infrastructures in Africa. It will highlight possible synergies among scientific unions and other bodies, like UNESCO and ICSU, to facilitate this process.
Crystallography in emerging nations: LACA
- Where: MR G.02-G.03
- When: Friday, 25 August, 15:30-17:30
- Chairs: Diego Lamas (Argentina, President of LACA), José Reyes Gasga (Mexico, Vice President of LACA)
- Speakers: Marcia Fantini (Brazil), José Reyes Gasga (Mexico), Mauricio Fuentealba (Chile), Leopoldo Suescun (Uruguay), Andrea Araya (Costa Rica), Sebastián Klinke, Adriana Serquis (Argentina), Graciela Diaz de Delgado, Miguel Delgado (Venezuela), others to be confirmed.
The Latin American Crystallographic Association (LACA), founded in October 2013, was recognized as a Regional Association of the IUCr during the 22nd IUCr Congress and General Assembly held in Montreal, Canada, in August 2014. At present, seven countries are full members of LACA: Argentina, Brazil, Mexico, Chile, Costa Rica, Uruguay and Venezuela. However, other countries already have active research groups in Crystallography and will probably organize national associations soon. In this context, the aim of this session is to analyze the present situation of Crystallography in Latin America and to discuss future actions to promote the growth of Crystallography in the region and the strengthening of the LACA.
Crystallography in emerging nations: the role for development in the BRICS countries
- Where: MR G.02-G.03
- When: Saturday, 26 August, 15:00-17:30
- Chair: Andreas Roodt, Jean-Paul Ngome Abiaga
- Lead Speakers: Marcia Fantini (Brazil), Mikhail V. Kovalchuk, A. F. Blagov (Russia), Ashwini Nangia (India), X.-D. Su (China), Susan Bourne (South Africa).
This session will present general activities from selected groups in the five BRICS countries (Brazil, India, Russia, China and South Africa) and will discuss initiatives on networking. It will also aim to increase collaboration and stimulate mutual discussions also among young colleagues, as well as funding opportunities available.
Saturday 26 August; 14.55 to 17.30: CHAIRS: J.-P.Ngome-Abiaga and A. Roodt
| Time for talk | Time slot | Speaker(s) | Title |
| 5-10min | 14.55-15.05 | A. Roodt (UFS, South Africa) and J.-P.Ngome-Abiaga (UNESCO, France) | Brief Introduction; Opening comments |
| 15min | 15.05-15.20 | M. Kovalchuk (Kurchatov Institute, Moscow, Russian Federation) | Crystallography as a Methodological Basis for Nature-Like Technology |
| 15min | 15.20-15.35 | M.C.A. Fantini (Campinas, Brazil) | Scientific Opportunities in Brazil |
| 15min | 15.35-15.50 | A.F. Blagov (RAS, Moscow, Russian Federation) | Complex Research at the Kurchatov Synchrotron-Neutron Facility |
| 15min | 15.50-16.05 | X.-D. Su (Peking University, Beijing, China) | Perspectives from China on the BRICS initiative |
| 15min | 16.05-16.20 | S. Bourne (Unv of Cape Town, South Africa) | The role of crystallography in a developing economy: South Africa |
| 15min | 16.20-16.35 | A Nangia(CSIR-NCL Pune, India) | Perspectives from India on the BRICS initiative |
| 10min | 16.35-16.45 | Y.A. Dyakova (Kurchatov Institute, Moscow, Russian Federation) | A New Approach to Finding Protein Crystal Growth Conditions |
| 10min | 16.45-16.55 | B.K. Saha (Pondicherry Univ, Puducherry, India) | Can hydrogen bond dimensionality be used to control thermal expansion in organic solids? |
| 10min | 16.55-17.05 | D.V. Kama, P.P. Mokolokolo, O.T. Alexander (UFS, South Africa) | A South African PhD Student Perspective on the BRICS Initiative |
| 20min | 17.05-17.25 | Round Table (Young students included; D. Kama, P. Mokolokolo, O. Alexander; and M. Elmakki, A. Belay); as well as young people from Russia, India, Brazil, China? | 1. Perspectives from Students and Young researchers 2. Network establishment supported by established and senior researchers |
| 5min | 17.25-17.30 | Concluding remarks | Brief conclusion |
Commemorative symposium in honor of Howard Flack
- Where: MR G.01
- When: Friday, 25 August, 10:30-13:00
- Chairs: Bill Clegg, Gervais Chapuis
- Speakers: Mike Glazer, Brian McMahon, Jordi Benet-Buchholz, Gervais Chapuis

This symposium will recognise and commemorate some of the diverse and important contributions made to crystallography by Prof. Howard Flack, who passed away earlier this year. Mike Glazer will recall memories of Flack’s early career in the research group of Kathleen Lonsdale. Brian MacMahon will document valuable contributions made by Flack to the development of online platforms and material of the IUCr. Jordi Benet-Buchholz will reflect on Flack’s research into aspects of chirality and absolute structure determination, including the ‘Flack parameter’. Gervais Chapuis will draw together contributions by Flack to crystallography research and teaching in Switzerland as recognised by his colleagues in that country.
Howard: the early years
Professor Mike Glazer, Oxford
In this talk I shall recall our first meeting and subsequent work together on disordered crystals at University College London under the guidance of Kathleen Lonsdale. I shall show some examples of Howard’s evident mathematical ability which later led him to make significant contributions to crystallography.
Howard’s giant leap for a chiral world: The Flack Parameter
Dr Jordi Benet-Buchholz, Tarragona
Several biological processes take place in a defined stereochemistry so that we can generally say that life, and of course human life, developed in a chiral world. Determination of the stereochemistry of molecules is of crucial importance when trying to correct biological processes that generate disease, affliction and illness. Howard Flack provided an elegant and enormously popular solution to the problem of absolute structure determination with a refinable parameter universally known as the Flack parameter. Since his contribution in 1983 he continuously revised the processes related to reporting and evaluating absolute configuration determinations until finally he published in a contribution with Simon Parsons a reorganization of the algorithm which
resolved observed inconsistencies.
Howard’s web
Brian McMahon, IUCr Chester
Howard Flack was a pioneer in using online tools to communicate between scientists and as a vital information dissemination system, initially predating the Worldwide Web then quickly becoming an enthusiastic early adopter. As Chair of the IUCr’s Electronic Publishing Committee, he was a strong leader in a number of initiatives that established crystallography as a scientific discipline leading the field during the information and data revolution.
Howard’s international career in Switzerland
Professor Gervais Chapuis, Lausanne
In 1972 Howard moved from Great Britain to take a software specialist position in the Institute of Crystallography at the University of Geneva. This was the start of a brilliant international career where Howard showed that he had deep knowledge in the computational aspects of crystallography, and in a sense continued in Switzerland the tradition that he acquired at University College in London during his PhD training. Howard’s interests included not only the field of absolute chirality but also the development of his famous Camel Jockey absorption software, and least-squares restraints for the origin fixing of polar space groups. He was the main contributor to the two IUCr reports on statistical descriptors published by an ad hoc committee. In addition the particular details which led Howard to the development of his famous parameter will also be presented.
Opportunity for brief contributions from the audience
Symposium in honor of William L Duax

- Where: MR G.01
- When: Saturday, 26 August, 15:00-17:30
- Chairs: Dr. S Narasinga Rao
- Speakers: Anthony Addlagatta, Susan K Byram, Hanna Dabkowska, Gracia Diaz Delgado, Claude Lecomte, Alex McPherson, S Narasinga Rao and D Velmurugan.
The microsymposium will be devoted to the childhood, early life, hobbies and scientific achievements of Prof. Bill Duax by his friends, colleagues and students across the globe. The speakers will be from Canada, France, India, USA and Venezuela.
Chair:
-
Dr. S Narasinga Rao, USA will speak on Prof. Duax’s childhood, early education, extracurricular activities, hobbies, scientific achievements and associations with notable world crystallographers. Further, he will discuss Duax’s interest in spreading crystallography throughout the world and his contributions to American Crystallographic Association (ACA), International Union of Crystallography (IUCr) and special interest in the future generation of youngsters.
Speakers:
-
Ms Susan K Byram, USA will talk on Prof. Duax’s distinguished career as a scientist and his contributions. She will focus on Duax’s commitment to crystallography in Asia, South America and Africa. She will also talk on his vision for the future of crystallography worldwide. His celebration of women and minorities in science, making crystallographic meetings friendly and fun, and entertaining the meeting attendees will be highlighted.
-
Dr. Claude Lecomte, France will focus on Prof. Duax’s contributions to science, crystallography, emerging countries and the IUCr.
-
Dr. Gracia Diaz Delgado’s, Venezuela, talk would include her first meeting with Prof. Duax in ACA meeting in 1987 and her continued interaction with him since then. In this talk, she will present Duax’s visit to Latin America and his contribution to crystallography in that region.
-
Dr. Hanna Dabkowska, Canada, will talk about Prof. Duax as a friend, his relations with all women in science who he was supporting during his career and his devotion to crystallography, teaching and photography. The title of the talk would be “Panegyric to Bill”.
-
Dr. D Velmurugan, India will speak on his connections with Prof. Duax since 1983 and Duax’s contributions to ACA newsletter, ‘fastest walker’, his dynamic personality and socializing. Duax’s contributions to stereochemistry, atlas of steroid structure and trends in hormone research and many other publications will be highlighted.
-
Dr. Alex McPherson, USA will offer testimonials to Prof. Duax’s colleagues and friends in the vast crystallographic community for the distinguished, yet always humble Bill.
-
Dr. Anthony Addlagatta, India will give a talk which will be more on a personal note about ‘Science and Life: Experience in Bill’s Laboratory and with Bill’s family.’
Dragon’s Den:
Supported by:
- Where: MR G.01
- When: Thursday, 24 August, 15:00-17:30; 2nd session on Saturday, 26 August, 10:30-13:00
- Dragons (selection panel): Ashwini Nangia, Massimo Nespolo, Amit Sharma, Christian Lehmann, Keith Moffat
- Sponsors: IUCr2017, STOE, Elsevier, Springer
Young researchers with maximum age of 35 and no permanent academic position, regularly registered for participation in IUCr2017, will present and defend their idea for a scientific project in front of the panel. Decision on what projects are funded is taken immediately at the end of all presentations. Awardees receive a grant of INR 1,50,000.